Vacuum
Information guide
Vacuum is the term commonly used to designate the state of a rarified gas (or gas/vapour mixture) charactarized by a pressure lower than the atmospheric pressure. There are 3 vacuum ranges :
- The primary vacuum, from 1000 to 10-3 mbar
- The secondary vacuum, from 10-3 to 10-7
- The ultra-high vacuum (UHV), less than 10-7 mbar
_1629967037.jpg)
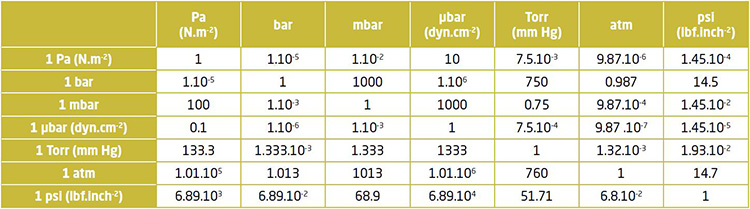
Below is the conversion table for units commonly used in Vacuum :
What is the purpose of vacuum ?
We live in an atmosphere considered as empty but which is in fact a environment made up of a very large number of air molecules, i.e. a majority mixture of nitrogen and oxygen. The concentration of molecules does not allow the implementation and the realization of certain processes currently used in industry. In order to carry out these processes, the molecular concentration in a given space must be lowered : this is what is called "creating vacuum". The applications using vacuum are numerous, in research as well as in industries.
Vacuum :
If the gas molecules were not attracted by the mass of the Earth, their incessant and rectilinear movements between 2 collisions would allow them to escape into space: the Earth's atmosphere would have disappeared long ago. The volumetric number of molecules and therefore the pressure decreases with altitude. Up about to 50km, the decrease follows an exponential law that Newton predicted: p=1.013 x 105 exp-(h/7500), p in Pascal and h in meters. Beyond that, the decay is much slower. The pressure drops and the nature of the gases changes (see table below).
_1629967046.jpg)
Fondamental vacuum values:
The pressure of 10-10 Pa seems to be the ultimate value currently achieved, but pressures close to 10-8 Pa are commonly reached in many installations. The magnitude of the pressure variation achieved should be noted : reaching 10-4 Pa means keeping only one molecule out of a billion initially present. This is a very exceptional technological feat, both in the field of obtaining and measuring. It is very important to define the range of vacuum needed in order to choose the most appropriate means to obtain it. Even in very high vacuums, there are several million molecules per cm3. This number is sufficient for the Maxwell-Boltzmann laws of statistical physics and the law of velocity distribution to remain valid. It is no longer the same in the interspace, if not at the scale of the dimension of these same spaces. In the intergalactic vacuum, there is only one molecule for 4m3. We could be tempted to consider that the absolute vacuum exists on the scale of the m3, but because of the speed of molecules, each m3 is crossed by more than 100 molecules each second. The absolute vacuum lasts only one hundredth of a second.
In order to make that particles (atoms, ions or electrons) moving in an installation are not disturbed by collisions with the residual gas molecules, it is necessary that the vacuum is close to 10-3 Pa. This is the level of vacuum that must be reached in machines designed to carry out vacuum deposition or devices operating with electron beams.
In order to avoid any pollution of a surface, on the scale of a monolayer, and this for duration of several minutes, it is necessary to work in ultra-high vacuum (UHV): that is to say pressures lower than 10-7 Pa. This is the case for surface analysis, as well as for deposition at very low speed, atomic plane after atomic plane in molecular beam epitaxy (MBE).
_1673427121.png)













